Many upper-school science teachers are aware that last year (2019) a substantial revision to the SI unit system, commonly known in the US as the metric system, went into effect. If you are not aware that the SI system underwent a significant revision, you should be.
The revision completely changed the definitions of four of the seven SI base units and four of the important and related physical constants. Minor formal changes were made to the other three base units.
The Major Changes
We have known these changes were coming. Four Novare Science texts (physics, chemistry, and physical science) call attention to the pending change to the definition of the kilogram, the only base unit still defined by a physical artifact—the International Prototype Kilogram (IPK). That is one of the changes that has taken effect. The IPK has now been retired.
Another important change is that several physical constants, the values of which were determined experimentally in the past, have now been defined as having specific, exact values. The one that affects high school science the most is Avogadro’s number, because of its relation to the definition of the mole—a unit used ubiquitously in chemistry. Formerly, we said that a mole of a substance was the amount of the substance that contained as many particles as there are atoms in exactly 12 grams of carbon-12. We also said that a mole was the amount of substance that contained Avogadro’s number of particles. With the 2019 SI revision, the first of these definitions is no longer valid. Avogadro’s number has now been assigned an exact value, and the official definition of the mole is that a mole of a substance is the amount of substance that contains Avogadro’s number of particles. Unfortunately, but unavoidably, the link between the mole and the kilogram has been broken. (Put another way, the molar mass constant, mu, is no longer equal to exactly 1. Happily though, it is close enough for all practical purposes, so our calculation methods do not change.)
At first, it can sound strange that an exact value has been assigned to something we are accustomed to thinking of as a physical constant that must be measured, but it’s really all about how we define our measurements. It helps to recall that this has happened before. The speed of light in a vacuum used to be something we tried to measure with greater and greater accuracy. But in 1983, the speed of light was assigned the exact value of 299,792,458 meters per second. Doing this changed the definition of what a meter is, and the meter became the distance light travels in 1/299,792,458th of a second.
This example with the speed of light and the meter is pretty easy to understand, so I keep it in mind when I am trying to wrap my brain around the others that are more difficult to understand. The kelvin used to be defined in terms of the absolute temperature of water at its triple point (273.16 K, equal to 0.01°C). It is no longer. Now, the value of the Boltzmann constant has been made exact, and the kelvin is now defined “by taking the value of the Boltzmann constant kB to be 1.380648 × 10–23 J/K.” It is more difficult to wrap your brain around how this defines the kelvin than it is to understand that fixing the speed of light defines the meter, but we’ll get used to it. Similarly, the kilogram is now defined by assigning Planck’s constant an exact value. (The connection in this case incorporates the Planck relation, E = hf, and Einstein’s mass-energy equivalence, E = mc2.)
Here is a summary of the major changes:
- Four physical constants have been assigned exact values: Planck’s constant (h), Boltzmann’s constant (k or kB), the elementary charge (e, the charge possessed by a proton or electron), and the Avogadro constant (NA).
- Four SI base units have been completely redefined:
- kilogram—formerly defined as the mass of the International Prototype Kilogram, now defined by setting an exact value to Planck’s constant.
- kelvin—formerly defined so that the temperature at the triple point of water would be 273.16 K, now defined by setting an exact value to Boltzmann’s constant. (Thankfully, the triple point is still at 273.16 K!)
- mole—formerly defined as the amount of the substance that contained as many particles as there are atoms in exactly 12 grams of carbon-12, now defined as an amount of substance containing Avogadro’s number of particles, with Avogadro’s number given an exact value.
- ampere—formerly defined in terms of the force between two current-carrying wires, now defined (very sensibly) as a current of one coulomb of charge per second, where the coulomb is defined as an amount of charge equal to the elementary charge, which has now been given an exact value. (Note for upper-level physics teachers: The former definition allowed μ0, ε0, and Z0 to have exact values related to μ0 = 4π × 10–7 H/m. These three constants are no longer exact.)
- The relationships between the seven base units and the seven relevant physical constants—which ones depend on the others—have changed. I find the two graphics below to be most helpful in thinking about these relationships. Note how the dependencies of unit definitions upon other units flows from top to bottom in the new SI system. The second affects everything but the mole, the meter affects three other units, the kilogram affects two others, and no definition depends on the definition of the four units at the bottom. Note also that the three physical constants not included in the 2019 revision have already been defined with exact values; the new changes make the whole system more consistent—all seven base units are now defined in terms of one or more physical constants that have been defined with exact values.
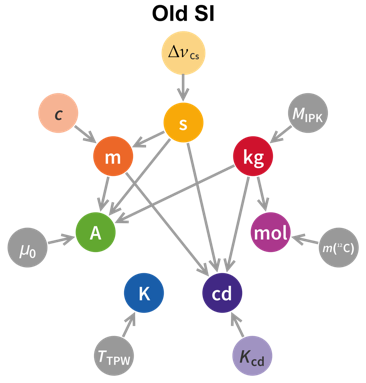
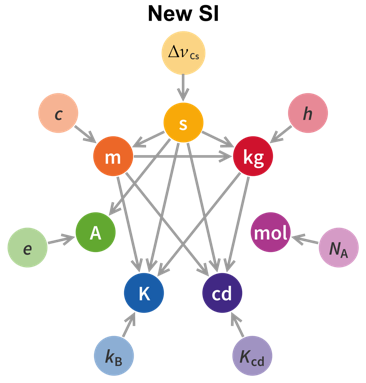
The new system makes a lot more sense, so even though we must now change our books, lesson plans, quiz and test keys, and memories, it’s all for the good.
How Does the New SI Affect Your Classes?
For those teaching with textbooks from Novare Science, the accompanying table indicates the major items affected by the 2019 revision to the SI system.
| Text | Items Affected |
| Novare Physical Science |
|
| Earth Science | Not affected. |
| Introductory Physics
Accelerated Studies in Physics and Chemistry |
|
| General Chemistry
Chemistry for Accelerated Students Physics: Modeling Nature |
|
| General Biology |
|
What’s the Plan at Novare Science?
The revisions to the metric system are a good thing. The system is more consistent. More importantly, there are no longer base units defined by an artifact, so the massive expense of calibrating secondary standards against a primary standard located in a vault somewhere has been eliminated. Even more importantly, laboratories in developing counties may now develop calibration procedures on their own, without the need to involve one of the countries who possess copies of the IPK. This is a victory for equity.
Over the coming weeks and months, we will be preparing revisions to our texts based on the 2019 SI revisions. Classical Academic Press will publish new editions for the texts (except Earth Science) in the coming years. In the meantime, we hope to prepare supplements for teachers to use for chemistry classes and with Physics: Modeling Nature. For the 2020-2021 school year, the accompanying table should get you by.
